Luer Lock Rings Pull-Off Force
ISO 11040-4 Annex G3
In the pharmaceutical and medical device industries, the luer lock rings pull-off force is a critical parameter to ensure the reliability and safety of prefilled syringes. Proper testing verifies the secure attachment of the luer lock adaptor (LLA) to prevent accidental detachment during use.
Importance of Luer Lock Rings Pull-Off Force
The luer lock rings pull-off force measures the axial strength required to detach the LLA collar system from the syringe barrel. Accurate testing ensures the syringe maintains integrity during handling, filling, and administration, reducing the risk of device failure. Regulatory standards mandate these tests to protect patient safety and support product performance claims.
For manufacturers and quality inspection agencies, especially those serving medical, pharmaceutical, and healthcare markets, mastering this test is essential. Cell Instruments provides advanced solutions that meet these stringent requirements with high precision and reliability.
ISO 11040-4 Annex G3 Requirement
ISO 11040-4 defines the requirements for glass barrels for injectables and sterilized subassembled syringes ready for filling. Specifically, ISO 11040-4 Annex G3 details the method for measuring luer lock rings pull-off force.
According to Annex G3:
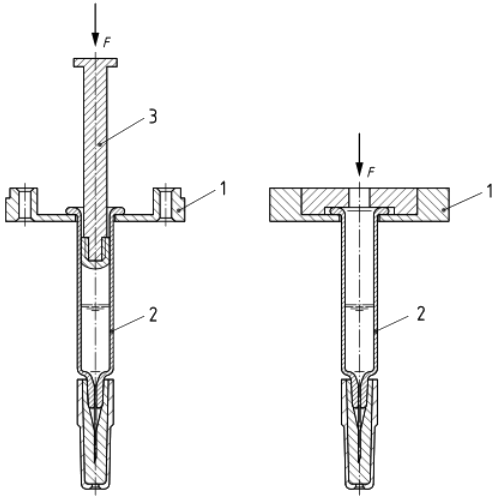
Principle: The test evaluates if the LLA system can resist a defined axial pull-off force when connected to a standard luer fitting.
Apparatus: A universal tensile and compression testing machine is required, capable of operating at a test speed of 20 mm/min. A load cell with a typical range of 10 N to 100 N must be used.
Preparation: Test samples must match the production process conditions.
Procedure: After removing the tip cap, the syringe is positioned in a holder without applying pre-pressure to the LLA. The machine then pulls at a constant speed until the LLA collar detaches, recording the peak force.
Results: The peak force from the force-displacement curve represents the pull-off strength.
Documentation: The report should include test speed, peak value, number of samples, pass/fail results, and any deviations observed.
Following ISO 11040-4 Annex G3 ensures that results are reliable, reproducible, and internationally accepted.
How to Perform the Luer Lock Rings Pull-Off Force Test
Conducting an accurate luer lock rings pull-off force test involves several caritical steps:
1. Sample Preparation
- Use sterilized subassembled syringes that mirror production conditions.
- Remove the tip cap without stressing the LLA collar system.
2. Setting Up the Test
- Mount the syringe in the syringe holder and secure the LLA collar in the gripper device.
- Ensure no pressure is exerted on the collar during setup.
- Calibrate the load cell to “zero” without applying preload.
Executing the Test
- Set the machine speed to 20 mm/min.
- Start the pull test, monitoring force versus displacement.
- Record the peak force once the LLA collar detaches from the syringe.
Analyzing the Results
- Identify the highest point on the force-displacement graph.
- Compare it with specified limits to determine pass or fail.
- Document all parameters, including sample quantities, failures, and deviations.
A sampling rate of 500 Hz is recommended to ensure the capture of accurate peak measurements during testing.

Recommended Equipment for Accurate Testing
At Cell Instruments, we specialize in providing universal tensile and compression testers customized for syringe testing applications. Our systems offer:
High-frequency sampling (up to 500 Hz) for peak accuracy.
Custom syringe holders and LLA grippers compliant with ISO specifications.
Load cells suited for a range of testing forces between 10 N and 100 N.
Intuitive software for full parameter traceability and easy reporting.
Our solutions guarantee compliance with ISO 11040-4 and help ensure the highest product quality for our customers in the pharmaceutical and medical device industries.
Understanding and accurately measuring the luer lock rings pull-off force according to ISO 11040-4 Annex G3 is crucial for ensuring syringe safety and reliability. By following the correct methods and using compliant, high-precision testing instruments from Cell Instruments, quality control teams can confidently verify product performance, meet regulatory standards, and protect end-users.
If you need assistance in selecting or customizing the right testing equipment for your syringe testing needs, contact Cell Instruments today to explore our full range of solutions.