LLA Collar Torque Resistance
ISO 11040-4 Annex G4
In pharmaceutical manufacturing, ensuring the mechanical integrity of prefilled syringes is critical to product safety and performance. One key factor in this integrity is LLA collar torque resistance, which measures the strength of the Luer lock adaptor (LLA) collar under torque. This property ensures that the collar can withstand the rotational force when medical personnel attach needle hubs or compatible connectors. Conducting this test in accordance with ISO 11040-4 and Annex G4 helps manufacturers meet regulatory compliance and deliver safe, functional products.
LLA Collar Torque Resistance and It Matters
LLA collar torque resistance refers to the capacity of the Luer lock adaptor collar on a syringe to resist turning or loosening when torque is applied. In real-world use, this simulates the mechanical stress exerted when a clinician connects a Luer-lock-compatible device. Failure in torque resistance can lead to leakage, improper drug administration, or contamination—serious risks in clinical settings.
Syringe Testing Standard - ISO 11040-4 Annex G4
The ISO 11040-4 standard, titled Prefilled syringes — Part 4: Glass barrels for injectables and sterilized subassembled syringes ready for filling, outlines rigorous test methods to ensure product reliability. Annex G4 specifically details the method for assessing LLA collar torque resistance, making it indispensable for manufacturers involved in syringe testing.
Key highlights from Annex G4 include:
- Test Principle: Verifies if the LLA collar can withstand torque during attachment of a standard 6% Luer taper fitting.
- Recommended Sampling Rate: 500 Hz for peak measurements.
- Standard Rotation Speed: 20 rpm or as agreed upon.
- Measurement Output: Peak torque at collar movement initiation.
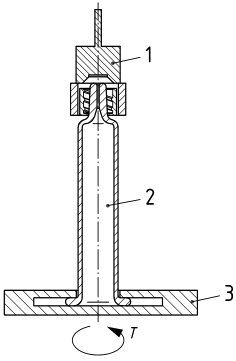
Test Equipment for LLA Collar Torque Resistance
Performing the test requires precision instrumentation. The standard setup includes:
- Torque Tester with Rotation Device: Should be capable of detecting peak torque with a resolution of 0.05 Ncm, ideal range being up to 35 Ncm.
- LLA Collar Gripper: Securely clamps the collar without damaging it.
- Rotatable Syringe Holder: Allows for accurate and consistent rotation of the syringe or the closure.
Step-by-Step Guide to Conduct the Test
Professionals in quality control can follow these steps to ensure accurate and standardized testing of LLA collar torque resistance:
- Insert the sterilized subassembled syringe into the syringe holder.
- Remove the tip cap manually.
- Mount the LLA collar gripper securely onto the LLA collar.
- Zero the torque cell to eliminate pre-load torque.
- Set the rotation speed to 20 rpm (or as appropriate).
- Initiate the test by rotating the syringe or closure 90° in the designated direction.
- Record the peak torque when collar movement begins.
This torque value reflects the maximum resistance offered by the LLA collar under operational stress.
Test Reporting and Quality Control
According to ISO 11040-4 Annex G4, a comprehensive test report must include:
- Peak torque values (Ncm)
- Rotation speed (rpm)
- Number of samples tested
- Pass/fail results
- Notes on deviations or anomalies
This documentation ensures traceability and compliance, both critical for regulatory audits and product release certification.
Advantages of Automated Torque Testing
At Cell Instruments, we specialize in precision test systems, including torque testers tailored for LLA collar torque resistance evaluations. Automation enhances:
- Measurement repeatability
- Data accuracy
- Operational efficiency
- Compliance assurance
Customized fixtures and software integration allow labs to streamline syringe testing workflows while conforming strictly to ISO guidelines.
Understanding and implementing the LLA collar torque resistance test in alignment with ISO 11040-4 and Annex G4 is vital for syringe quality assurance. This test safeguards against mechanical failure during clinical use and helps manufacturers uphold the highest standards of patient safety. At Cell Instruments, we provide state-of-the-art torque testing solutions that align with global standards and industry needs, empowering syringe manufacturers with reliable data and regulatory confidence.