Injectability Test
Syringe Functionality Testing
The injectability test is a crucial evaluation method used in the development and quality control of syringes and injection systems. It directly impacts patient comfort, drug delivery effectiveness, and regulatory compliance. By examining how easily and consistently a formulation can be delivered through a syringe, the test ensures the usability and performance of injectable products under real-world conditions.
For medical device developers, pharmaceutical manufacturers, and packaging engineers, understanding injectability testing is key to delivering safe and efficient drug delivery systems.
Syringe Functionality Testing - ISO Syringe Standards
To maintain safety and global regulatory compliance, several ISO standards define the framework for injectability and syringe functionality testing. These documents serve as industry benchmarks and are indispensable for product validation and batch release.
ISO 11040-4 Annex E: Prefilled Glass Syringe Performance
This standard addresses the injectability characteristics of prefilled glass syringes, including force required to initiate and sustain fluid flow, flow continuity, and absence of plunger stalling or leaking. Annex E specifies test parameters that simulate clinical use—like consistent plunger speed and controlled environmental conditions—to ensure the syringe performs effectively across scenarios.
ISO 7886-1 Annex E: Single-Use Hypodermic Syringes
Annex E of ISO 7886-1 focuses on injectability performance for disposable hypodermic syringes. Key evaluation metrics include:
- Break-loose and glide force
- Injection continuity and obstruction checks
- Plunger return after dispensing
These evaluations are essential for syringes used in hospitals, outpatient care, and at-home treatments.
ISO 8537 Annex C: Syringes for Insulin Injection
Injectability is especially critical in insulin syringes, where low viscosity fluids and small dosages demand ultra-smooth delivery. ISO 8537 Annex C emphasizes force consistency and manual handling accuracy, ensuring that the injection can be administered by users of various skill levels, including patients themselves.
ISO 11499: Compatibility and Performance of Injector Systems
For systems that integrate syringes into injector assemblies, ISO 11499 defines criteria for mechanical compatibility and injectability performance. The standard considers factors like plunger integrity, injection time, and total dose delivery, especially under dynamic force conditions.
ISO 11608-3: Needle-Based Injection Systems
ISO 11608-3 outlines testing strategies for automated injectors, pen systems, and wearable devices. Injectability here involves more complex mechanisms—plunger actuation, drug viscosity adaptation, and multi-phase delivery. The standard demands high-precision injectability testing that mimics auto-injection behavior and environment-specific variables.
Injectability Test Parameters and Methodology
Injectability is not a single attribute but a combination of measurable physical parameters that reflect user experience and mechanical performance. Key test variables include:
- Break-loose force: The force required to initiate movement of the plunger
- Glide force: The force necessary to maintain consistent plunger motion
- Delivery time: Time needed to expel the complete dose
- Flow consistency: Evaluates interruptions or resistance during delivery
- Backpressure behavior: Indicates syringe and fluid compatibility
Test conditions often simulate clinical injection speeds, specific angles, and environmental factors like temperature or humidity. The objective is to mirror actual usage as closely as possible to detect any potential performance issues before the product reaches patients.
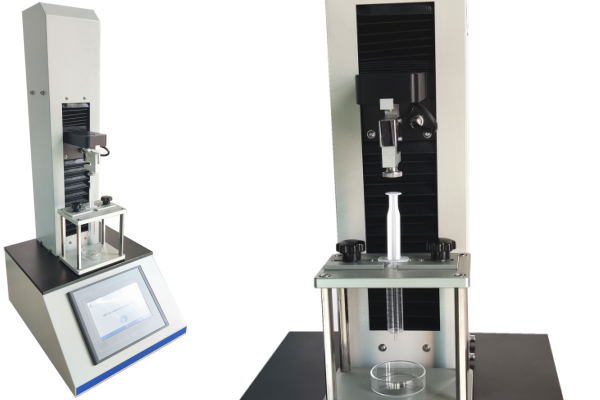
Test Solutions from Cell Instruments
Cell Instruments Co., Ltd. provides advanced solutions tailored to injectability test needs. Our Syringe Injectability Tester is engineered to support fully programmable testing cycles, including plunger speed control, force measurement, and injection simulation based on ISO standards.
Key features include:
- Compatibility with ISO 11040-4, ISO 7886-1, ISO 8537, ISO 11499, and ISO 11608-3
- Digital force sensors for high-resolution break-loose and glide force detection
- Support for both prefilled and manually filled syringes
- Easy integration into R&D labs and production QA workflows
With options for customization and automation, Cell Instruments helps customers achieve faster throughput, reduced variability, and greater test confidence.
Applications and Practical Insights for Manufacturers
The injectability test is vital for:
- Formulation development: Ensuring that viscosity, particulates, or excipients do not impede syringe operation
- Packaging compatibility: Verifying that barrels, plungers, and seals allow smooth delivery
- Regulatory compliance: Meeting ISO and FDA expectations for injectable medical devices
- User-centered design: Making injection easy and comfortable for patients and clinicians
Failing injectability assessments can lead to product recalls, patient safety issues, or regulatory delays. Incorporating standardized, high-fidelity testing from the early stages of design reduces these risks and builds stakeholder trust.
Why Cell Instruments is the Right Choice
Cell Instruments combines decades of expertise in material and device testing with a strong focus on syringe functionality testing. We serve pharmaceutical companies, syringe manufacturers, biotech firms, and quality inspection agencies across global markets.
- Custom-built injectability testing solutions
- Strict alignment with international standards
- Expert technical consultation and responsive support
- Automation options to streamline test efficiency
Whether you’re validating a new injector system or scaling up production of prefilled syringes, Cell Instruments ensures your injectability data is reliable and regulation-ready.