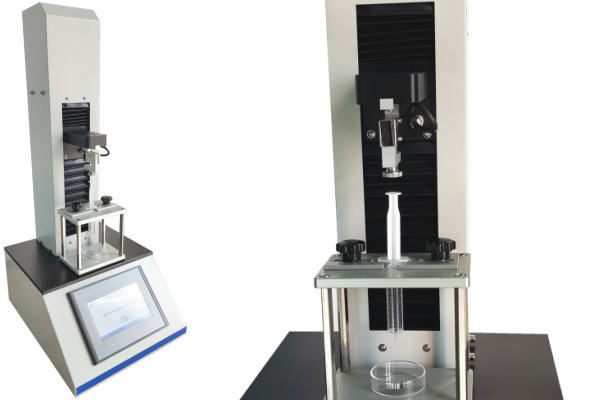
Syringeability Test
Syringe Functionality Testing
Ensuring that a syringe performs reliably during actual use is critical in both clinical and laboratory environments. The syringeability test evaluates a syringe’s ability to deliver its contents smoothly, safely, and consistently. More than a simple flow assessment, it also considers factors such as injection force, obstruction, ease of administration, and compatibility with various substances.
Importance of Syringeability Testing
The syringeability test ensures that syringes deliver drug products without clogging, excessive force, or loss of dose. For drugs in prefilled syringes, biologics, or viscous formulations, syringeability becomes even more critical.
Pharmaceutical manufacturers, R&D professionals, and QA/QC teams rely on these tests to validate product design, packaging, and patient usability. Poor syringeability can lead to patient discomfort, underdosing, or failed delivery—risks that regulatory authorities strictly monitor.
Relevant Standards for Syringe Functionality Testing
To ensure compliance and consistency, multiple ISO standards outline the procedures for syringe functionality and syringeability evaluation. Each provides a specific context for different syringe types and applications.
Syringe Functionality Testing per ISO 11040-4 Annex E
ISO 11040-4 focuses on glass barrels for prefilled syringes. Annex E outlines a syringeability test to verify functional performance, emphasizing:
- Plunger force consistency
- Break-loose and glide force measurement
- Absence of leakage or stalling
- Compatibility with viscous or particulate-filled solutions
Testing involves precise control of injection speed, force, and volume to simulate real-life scenarios.
Syringeability Criteria in ISO 7886-1 Annex E
ISO 7886-1 applies to sterile hypodermic syringes for single use. In Annex E, the syringeability test examines:
- The force required to expel the liquid
- Smoothness and continuity of plunger movement
- Ease of administration over various conditions
This standard is critical for disposable syringe manufacturers looking to meet international quality benchmarks.
High-Volume and Specialty Device Testing: ISO 8537 Annex C
For insulin syringes, ISO 8537 Annex C presents syringe functionality test parameters tailored to low-dose precision and patient-administered injections. The standard evaluates:
- Injection volume accuracy
- Syringe usability for patients with limited dexterity
- Force thresholds under real-use conditions
Device Compatibility in ISO 11499
ISO 11499 guides testing of single-use injectors for medical use, ensuring performance consistency and drug compatibility. Syringeability testing ensures that resistance and blockage do not hinder dose delivery, especially in injectors used for chronic diseases.
Autoinjector and Pen System Evaluation: ISO 11608-3
ISO 11608-3 details requirements for needle-based injection systems like autoinjectors and pen injectors. It includes:
- Assessment of syringe interaction with the delivery device
- Resistance to plunger deformation or force surges
- Suitability for use with a broad range of medications
Conducting the Syringeability Test: Parameters and Equipment
A successful syringeability test includes multiple factors:
- Break-loose force: the initial force to start plunger movement
- Glide force: force required for continuous motion
- Flow rate: measured at specified injection speeds
- Back pressure: resistance caused by formulation characteristics
Cell Instruments offers high-precision testing systems ideal for syringeability evaluations. Our NPT-01 Syringeability Testing Machine is engineered to comply with ISO 11040-4, ISO 7886-1, and ISO 11608-3. It supports programmable speed, plunger force, and injection simulation under controlled conditions, providing high reproducibility and accuracy.
Whether you’re testing prefilled syringes, biologic drugs, or innovative injector systems, Cell Instruments delivers customized syringeability test solutions for R&D, QA/QC, and production validation needs.
Best Practices for Reliable Syringeability Testing
To achieve consistent and regulatory-compliant results:
- Use standardized test fluids, particularly if testing for general functionality. For actual formulations, consider viscosity and particle content.
- Calibrate the instrument regularly to ensure force and displacement accuracy.
- Simulate realistic injection rates, such as those used in clinical settings.
- Document all parameters, including syringe type, formulation, ambient temperature, and test environment.
Additionally, integrating automation—such as robotic plungers or synchronized flow sensors—can improve repeatability and reduce human error.
Why Choose Cell Instruments' Syringeability Testing Machine
As a specialized manufacturer of Syringeability Testing Machine, Cell Instruments supports pharmaceutical and medical device customers with:
- Customizable testing solutions for diverse syringe types and formulations
- Compliance with global standards like ISO 11040-4, ISO 7886-1, and ISO 11608-3
- Expert technical support to help clients optimize their test setups
- Automation upgrades for higher throughput and reduced manual intervention
Our instruments are widely used by pharmaceutical labs, packaging developers, and regulatory compliance teams.