Softgel Quality Control for Consistent Performance and Safety
Softgel quality control sits at the core of pharmaceutical and nutraceutical manufacturing. Softgels must protect active ingredients, maintain stability throughout shelf life, and deliver a reliable patient experience. Any deviation in shell strength, elasticity, or texture can lead to leakage, deformation, inaccurate dosing, or consumer complaints. For this reason, manufacturers increasingly rely on objective mechanical testing to support formulation development, routine production monitoring, and regulatory compliance.
Softgel quality control starts with understanding how gelatin-based shells behave under compression, deformation, and repeated stress. Mechanical parameters such as hardness, elasticity, and structural recovery directly reflect formulation balance, plasticizer content, and encapsulation conditions. By transforming these physical properties into measurable data, quality teams gain control over product consistency instead of relying on subjective inspection.
Softgel Encapsulation Technology and Its Impact on Quality Control
Softgel encapsulation technology defines how gelatin, plasticizers, and active fills interact during forming, sealing, drying, and storage. Even minor changes in this process can influence shell thickness, seal integrity, and moisture distribution, all of which affect mechanical performance.
From a quality control perspective, softgel encapsulation technology determines what must be tested and when. Early-stage R&D focuses on optimizing gelatin formulation and elasticity. During scale-up, hardness and deformation behavior become critical indicators of process stability. In finished products, quality control verifies that each batch meets predefined acceptance criteria before release.
Mechanical testing bridges formulation science and manufacturing reality. Instead of only checking visual defects, laboratories now quantify how a softgel responds to compression, rupture, and recovery, ensuring that encapsulation technology delivers predictable results.
Mechanical Parameters in Softgel Quality Control
Effective Softgel quality control relies on a structured evaluation of key mechanical parameters. Among them, hardness, elasticity, and texture profile analysis stand out as the most informative indicators.
Softgel Hardness Test as a Core Indicator
Den softgel hardness test measures resistance to deformation or rupture under controlled compression. This test reveals whether the shell can withstand stresses during packaging, transportation, and handling. Excessive hardness may indicate over-drying or brittle shells, while insufficient hardness often signals weak seals or excessive plasticizer content.
Hardness testing also supports batch-to-batch consistency. By tracking hardness trends, manufacturers quickly identify process drift and correct issues before defects escalate.
Softgel Elasticity Testing for Structural Recovery
Softgel elasticity testing evaluates how well a capsule recovers its shape after deformation. Elastic behavior matters because softgels experience repeated mechanical stresses, especially in bulk packaging and distribution.
Elasticity data help quality teams understand the balance between flexibility and strength. Capsules that recover smoothly maintain seal integrity and appearance, while poor elasticity increases the risk of permanent deformation or leakage. This parameter proves especially valuable during stability studies, where aging can significantly alter shell behavior.
TPA Texture as a Comprehensive Mechanical Profile
TPA texture analysis, adapted from texture profile analysis methods, provides a deeper mechanical fingerprint of softgels. Instead of a single force value, TPA texture captures multiple attributes such as hardness, resilience, cohesiveness, and springiness in one test sequence.
In Softgel quality control, TPA texture helps correlate mechanical behavior with sensory perception and real-world performance. It also supports comparative studies between formulations, suppliers, or processing conditions, making it a powerful tool for both R&D and routine quality assurance.
Standards and Best Practices Supporting Softgel Quality Control
Although pharmacopeias do not always specify a single universal method for softgel mechanical testing, industry best practices emphasize controlled compression testing, repeatability, and traceable data. Mechanical analysis aligns with general pharmaceutical quality principles, including GMP requirements, data integrity, and validation.
Quality control laboratories follow standardized conditioning, defined test speeds, and consistent deformation settings to ensure meaningful comparisons. These practices strengthen confidence in test results and support regulatory audits by demonstrating scientifically justified control strategies.
Instrumentation for Reliable Softgel Quality Control
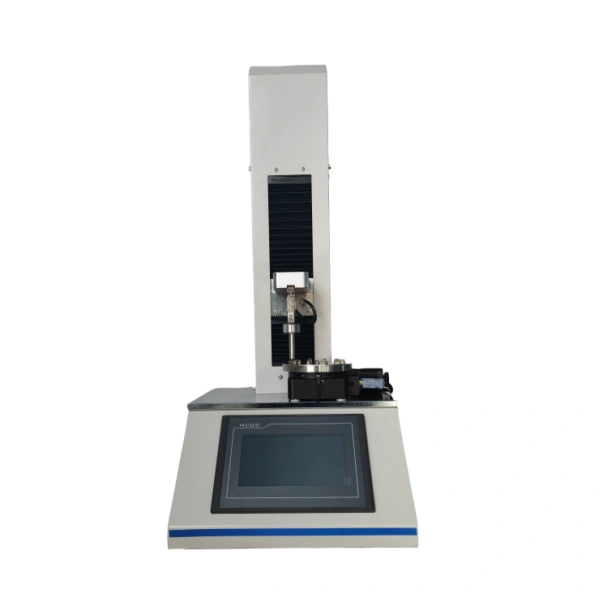
Accurate results depend on purpose-built instrumentation. Advanced capsule and softgel hardness testers provide controlled force application, precise displacement measurement, and flexible test modes. Such systems allow laboratories to perform softgel hardness test routines, elasticity evaluations, and TPA texture analysis on a single platform.
Den Cell Instruments Capsule and Softgel Hardness Tester supports comprehensive Softgel quality control by combining force, distance, and peak testing capabilities. Its precise motion control and customizable parameters help manufacturers simulate real handling stresses while maintaining high repeatability. This versatility makes it suitable for production QC, formulation research, and stability studies without duplicating equipment.
Benefits of Robust Softgel Quality Control Programs
A well-designed Softgel quality control program delivers tangible benefits across the product lifecycle. Manufacturers reduce batch failures by detecting mechanical weaknesses early. R&D teams accelerate formulation optimization with quantitative feedback. Quality managers gain confidence that each released batch meets safety and performance expectations.
Most importantly, consistent softgel quality protects patient trust. Capsules that look intact, feel consistent, and perform reliably reinforce brand credibility in highly competitive pharmaceutical and nutraceutical markets.
Konklusjon
Softgel quality control has evolved from basic inspection into a data-driven discipline grounded in mechanical testing. By integrating softgel hardness test methods, softgel elasticity testing, and advanced TPA texture analysis, manufacturers gain precise control over capsule performance. Supported by robust instrumentation from Cell Instruments, modern quality control programs ensure that softgel encapsulation technology delivers safe, effective, and consistent products to the global market.